Kelly Monk, PhD, is a big fish in the vast sea of glial cell biology. Monk, professor and co-director of OHSU’s Vollum Institute, is one of the world’s leading experts on the cells that produce myelin – the insulation around nerve cells that helps nerve impulses to move quickly and efficiently, much like insulation on a wire.
In human evolution, myelin is what allowed our ancestors and other large vertebrates grow to much larger sizes and develop complex central nervous systems. In modern humans, myelin damage is the key cause of most debilitating diseases of the brain and spinal cord.
Monk leads a lab team of 11, whose research has important implications for diseases of the central nervous system – multiple sclerosis for example – as well as for peripheral nervous system disorders such as painful, chronic neuropathies. Myelin loss appears to drive these conditions, and if scientists can figure out how to repair myelin, they may be able to find new treatments.
Successfully promoting myelin repair will be essential to cure nearly all injuries and neurodegenerative diseases of the brain and spinal cord.
Monk approaches this challenge as a basic scientist – guided by the belief that a better understanding of basic biology will reveal the mechanisms of human disease. She is interested in learning how neurons and myelinating cells interact, and how the vertebrate nervous system evolved after myelin appeared. Much of that understanding is thanks to a tiny fish.
Zebrafish: almost human

Behold the zebrafish. At less than two inches long, it’s hard to imagine that this tiny member of the minnow family would have anything in common with humans – or that it could hold the key to understanding how our brains and nerves work.
The zebrafish shares 70 percent of its genes with humans. Its immune system is incredibly similar to ours, and its neurons communicate with each other like human neurons. In fact, of all the genes that are associated with human disease, 84 percent have a counterpart in zebrafish.
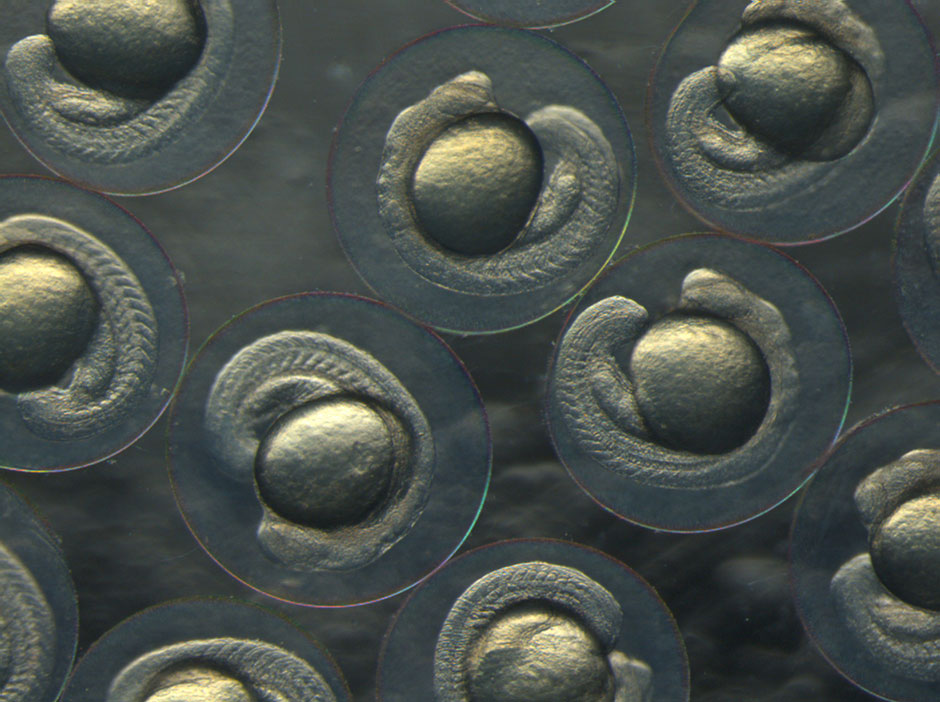
Zebrafish are among the most useful animals in neuroscience research, enabling studies that are more difficult or impossible with other species such mice. One key advantage is the ease with which scientists can manipulate the zebrafish’s genes to create mutations and abnormalities that mimic those found in human diseases. Also, unlike mice, zebrafish embryos develop outside the mother, with transparent skin that makes it easy to watch their organs (including the brain and spinal cord) develop in real time from conception. They mature and reproduce quickly, which is ideal for generational studies, and they also require less space than mice.
Most importantly for Monk’s work, zebrafish – like humans and all other jawed vertebrates – produce myelin. That means her team can exploit the impressive array of molecular-genetic and embryological tools available in zebrafish to understand fundamental principles of myelin biology.
Today the zebrafish is one of the most frequently used model organisms for genetic research, and it all started in Eugene in the late 1960s.
“The zebrafish research community in Oregon is among the best in the world,” Monk says.
Glial cells finally get respect
As a postdoctoral investigator at Stanford University, Monk helped to pioneer the use of zebrafish as a model for studying glial cell biology.
When we think about cells in the nervous system, we typically think of neurons – the cells responsible for sending electrical and chemical signals throughout the body. Neurons may be the cellular building blocks of the nervous system but they are not alone. Non-neuronal cells called glia represent at least half of the cells in the human brain.
Glia do not produce electrical impulses, but they support and insulate neurons – and send and receive signals to neurons and other glia. Glia were mostly ignored until the end of the 20th century because scientists thought they just passively took up space while neurons were busy zipping electrical signals along the body’s complex network of nerve fibers called axons. In the era of molecular genetics, that began to change as researchers discovered the critical functions that glial cells perform.
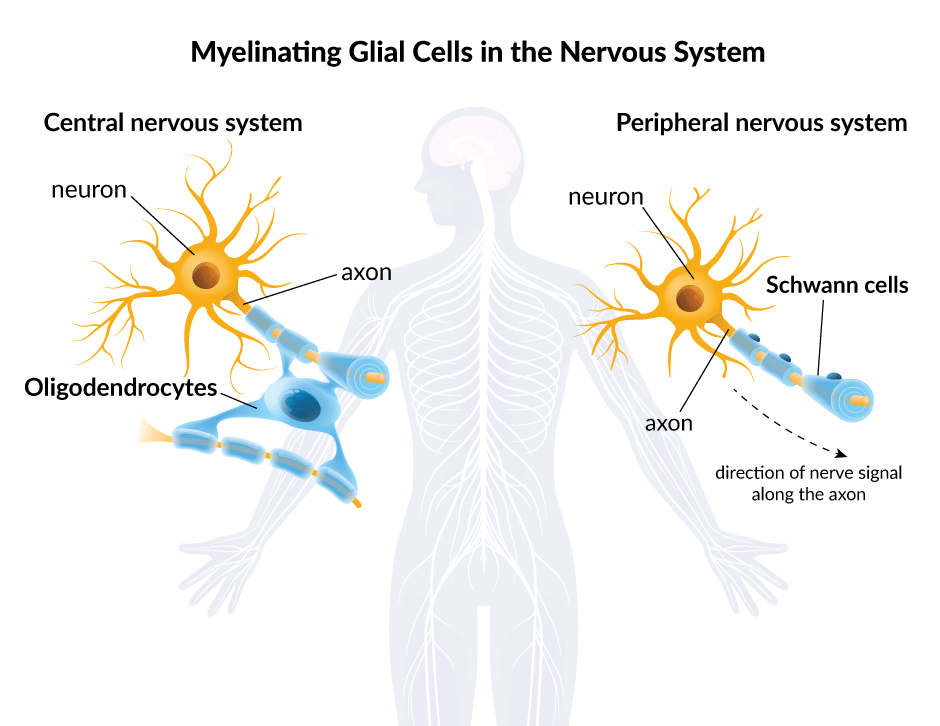
Glial cells come in many forms, but only two kinds produce myelin. Glial cells called oligodendrocytes create the type of myelin found in the brain and spinal cord – the chief components of the central nervous system. By contrast, the myelin that insulates the body’s peripheral nerves is formed by what are known as Schwann cells.
“Myelination” – the process by which myelin forms – has been studied for centuries but remained a mystery until Monk’s lab made several key breakthroughs.
“Kelly identified what many of us refer to as the holy grail of myelin biology when she discovered Gpr126, the receptor that seems to drive the formation of all myelin in the peripheral nervous system,” says Marc Freeman, director of the Vollum Institute and Monk’s colleague. “She later identified the equivalent receptor in the brain and spinal cord, which together were foundational contributions to the field.”
Monk’s lab continues to explore how the signals between glial cells and axons regulate myelination, and how myelin is maintained once it is formed. In the case of injury or disease, her team wants to learn whether the same developmental pathways that regulate myelination also regulate remyelination, or if there are additional pathways necessary. By learning to manipulate these processes, the team hopes to develop therapies to promote remyelination in patients with neurodegenerative diseases or injuries.
In a 2019 study published in the influential journal Nature Communication, Monk showed that Schwann cells could be much more prolific in generating myelin than previously believed. Before, scientists believed that only oligodendrocytes generated multiple myelin sheaths around axons.
“The field knew that Schwann cells only wrap around one axon, but it was not clear why that happens or how,” Freeman explains. “Kelly found the first piece of the mechanism because when she broke a certain gene, Schwann cells now wrapped multiple axons,” he says.
The discovery may lead to new gene therapy techniques to repair damaged myelin in peripheral nervous system disorders such as Charcot-Marie-Tooth disease, a painful inherited form of neuropathy that affects 1 in 2,500 Americans.
Even more promising: the study also suggested a new approach to healing the brain and spine by targeting the Fbxw7 gene to promote myelin repair in the central nervous system.
Collaborating all over the world – and OHSU
Monk did groundbreaking work first at Stanford and later at Washington University’s Department of Developmental Biology before she was recruited to OHSU in 2017.
Her impact on the field has been enormous, says James Salzer, MD, PhD, a leading glial cell biologist and professor in the Department of Neuroscience and Physiology at New York University Langone Medical Center.
“Dr. Monk has made a number of seminal contributions that have greatly advanced the field,” Salzer says. “Her studies identified receptors’ key roles in glial development and elucidated and directly connected decades of work in two separate areas of myelin research.”
As Monk has shifted into her role as co-director of the Vollum Institute, most of the day-to-day bench research in her lab has shifted to her team – a transition she finds highly rewarding.
“It’s really gratifying to see my trainees making those discoveries,” Monk says. And the research still excites her. “It’s putting the pieces of the puzzle together. How your discovery fits into what came before. And how each discovery not only solves part of the puzzle but also opens up 20 new doors that you never would have thought about.”
“It’s putting the pieces of the puzzle together. How your discovery fits into what came before. And how each discovery not only solves part of the puzzle but also opens up 20 new doors that you never would have thought about.”
Kelly Monk, PhD
Monk regularly collaborates with glial cell scientists around the globe. She was one of 22 scientists invited to present at a Nobel mini-symposium at Stockholm’s Karolinska Institute in 2019, and she will speak at a conference in Crete in 2020.
She is also part of a thriving community of glial cell researchers across OHSU – a glial cell research hotspot in the heart of zebrafish country. Freeman himself is a noted glial cell expert. Ben Emery’s lab in OHSU’s Jungers Center for Neurosciences Research is focused on glial cells in the central nervous system and the development and repair of oligodendrocytes. Mary Logan’s lab, also at the Jungers Center, studies the signaling pathways that control glial cell recognition. Anusha Mishra an assistant professor of Neurology in the School of Medicine, studies glial cells called astrocytes in the central nervous system. Bahareh Ajami, who recently joined the Department of Molecular Microbiology and Immunology, studies microglia – the brain’s intrinsic immune cells.
Salzer says Monk’s work has led to the identification of unexpected factors in myelination that other researchers in the field can build on.
“We are still in the early innings in glial research, and Kelly’s work underscores there are many more exciting discoveries yet to emerge.” He says she has a unique ability to move between mouse and zebrafish models, taking advantage of the best of each model, to accelerate her findings.
Hope for people with MS and myelin-related disorders
In the 20 years that Monk has worked in myelin research, all the available drugs for multiple sclerosis have targeted the immune system rather than myelin repair. However, she remains hopeful that scientists will soon learn to tap the therapeutic potential of myelin repair. Although she doesn’t want to make any promises that a new breakthrough drug is just around the corner, she says the pace of progress in recent years is encouraging.
“I think we’ve learned more in the past five years than we had in the 15 before that,” she says. “The rate of knowledge acquisition just seems exponential.”
Kelly Monk, PhD, receives support from the National Institute of Neurological Disorders and Stroke of the National Institutes of Health. Monk is a Harry Weaver Neuroscience Scholar of the National Multiple Sclerosis Society.
Dr. Monk’s photo credit: OHSU/Kristyna Wentz-Graff

